Mihir Shah asked for a lot of advice when the Food and Drug Administration initially rejected his company’s application to clear a new adjunctive breast cancer screening device last year. People told him to call a local politician. Lawyers suggested a range of motions as they billed $600 per hour. Some said get in line – thousands of other companies complain every day about how the FDA’s often unpredictable approval process.
One thing was clear:
“There was no way I was going to sit on my ass for two years,” says Shah, who joined the millions of people whose lives have been touched by breast cancer when his mother-in-law was diagnosed with it in 2007.
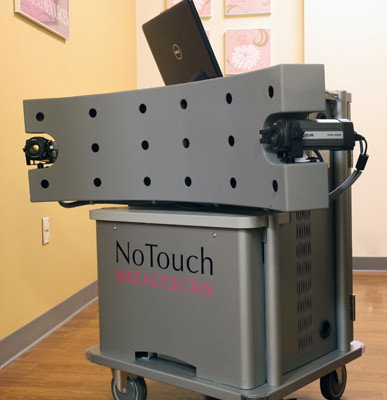
So Shah went straight to the source, writing a letter to Dr. Jeffrey Shuren, the FDA's Director for Devices and Radiological Health. To his surprise, Shah received a reply two weeks later. The FDA encouraged Shah’s company, UE Life Sciences, to resubmit its application, which it did last Nov. 4. Less than 100 days later on Feb. 10, the FDA cleared UE LifeSciences’ NoTouch BreastScan device and software, a new low-cost, non-invasive and radiation-free infrared technology that provides accuracy where mammograms have been proven to be less effective in detecting breast cancer.
Mammography’s Diminished Returns
While film mammography has long been the standard for breast cancer screenings, recent findings indicate it is relegated to near coin-flip accuracy for those women under 50 and those with dense breasts. A $5 million clinical study of 50,000 women across the U.S. from 2001-2005 and published in the New England Journal of Medicine revealed standard film mammography’s diminished accuracy in detecting breast cancer among those groups when compared to digital mammography.
Shah estimates there are up to 30 million women in the U.S. who have highly dense breasts (and thus could be three times more likely to be diagnosed with breast cancer) but are age ineligible for a mammogram because of their age. Insurance typically covers mammograms for women over 40, but not always.
“It seems that radiologists are understanding the hard way that mammography is an injured tool,” says Shah. “In fact, there is no silver bullet solution. The answer is to adopt a 'multi-modality' approach.”
According to Shah’s research, there are 13,000 mammography centers in the U.S. and only 400 U.S. clinics offering breast thermography. Improvements and affordability of digital cameras are making thermography a more realistic adjunct to traditional mammograms.
UELS's risk-free, 10-minute exam creates a thermographic map of the breasts in its search for signs of breast cancer. Incorporating computer software, the test analyzes very small changes in heat patterns at the pixel level and generates a report of its findings, eliminating the need for manual interpretation. It is the first device of its kind that uses a dual-infrared detector. The device can be used on women as young as 18 and as often as necessary. At about $150, it comes at a fraction of the cost of mammograms – which can run about $3,000 when not covered by insurance – making it a potentially widely available complement to existing tests.
Blazing His Own Trail
The FDA’s initial rejection, Shah contends, was based on supporting new FDA guidelines that advised women under 50 don’t need to get mammograms, relegating adjunct modalities like the NoTouch BreastScan to underdog status.
“In medical devices, there is no business plan if you don’t have FDA approval,” says Shah, whose company is a finalist for May's Greater Philadelphia Alliance for Capital and Technologies (PACT) Enterprise Awards in the Life Science Startup category.
“It’s kind of the global benchmark. It is amazing it took this long.”
The FDA clearance is a capstone (for now) of Shah’s 16-year journey that started in his native India and shifted to Philadelphia when he came to pursue his Bachelor’s in Computer Engineering at Drexel University in 1997. The latest in a long line of family entrepreneurs, Shah connected with like-minded innovators locally and started working in the IT space, winning third prize in a Drexel business plan contest in 2002 for OASIS (Online Appraisal Status Information System), a web tool that creates efficiencies for real estate appraisals. The team got to work at the Baiada Business Center, picking up invaluable support and management experience. While here, Shah got to learn about technology licensing from the nearby Drexel Technology Commercialization Office.
Soon he took interest in a brain imaging device that UPenn and Drexel teamed up on and found himself sitting next to a BBC journalist in India talking about its potential. This sparked his passion for medical devices, but Shah didn’t want to just work on others’ technology. In January, 2009, Shah founded UE LifeSciences with a small team and big goals.
He went about learning the science of infrared imaging, getting his father and a few others to invest $150,000 in a medical device company that had no product. In late 2010, UE licensed a tissue scanner for breast cancer developed by Drexel Associate Professor and breast cancer survivor Dr. Wan Shih. The device was included in the inaugural QED Proof of Concept Program at the University City Science Center and a lengthy quest for the FDA’s blessing had begun.
Pathways to Beating the Disease
Shah says his company’s goal for this year is to have its device in use at 100 gynecology, breast surgery and radiology clinics. Four clinics in Long Island are already committed to using it. Dr. Ari Brooks, a surgical oncologist who practices at Drexel Surgery in Center City, plans on being the first local practitioner to use the device. He likes the technology because of its reliability — two cameras, scientifically set at a distance to produce repeatable results. He says Drexel Medicine has agreed to set up a pod for NoTouch BreastScan at four sites in Center City and Manayunk that will be available at least once per week.
“Women are not walking in off the street saying 'I need a mammogram,'” says Brooks, who developed a virtual multidisciplinary breast center at the Hahnemann and Graduate Hospital sites that offer evaluation and care for breast cancer patients. Brooks is also an associate vice dean for research, technology and innovation for Drexel's College of Medicine.
“And utrasound is not a good test. Thermography is a very nice way for a woman who is concerned and does not meet the insurance criteria. Maybe she had a grandmother who had (breast cancer).
“This has the potential to give the consumer a little bit more control in the management of their own health until we take control when they meet the criteria.”
Globally, there is an even greater market, according to Shah. A large pharmaceutical company in India has already offered to purchase 25 of UELS’s systems. Shah cites India – where he knows nine women in addition to his mother-in-law who have been diagnosed with breast cancer despite living otherwise healthy lives – Russia, Asia and the Middle East as prime markets where mammography and breast cancer awareness in general are less prominent.
Shah says UELS hopes to pursue a web-based platform that will streamline the remote administration and reporting of testing.
“This has to soon become a mainstream medical tool,” says Shah, whose company is seeking Center City office space for its currently virtual effort. “Whatever we have to do as a medical device company to enable that is what we are going to do.”
JOE PETRUCCI is managing editor of Keystone Edge. Send feedback here.